Interactions between hydrated cerium(III) cations and carboxylates in aqueous solution: Anomalously strong complex formation with diglycolate suggesting a chelate effect
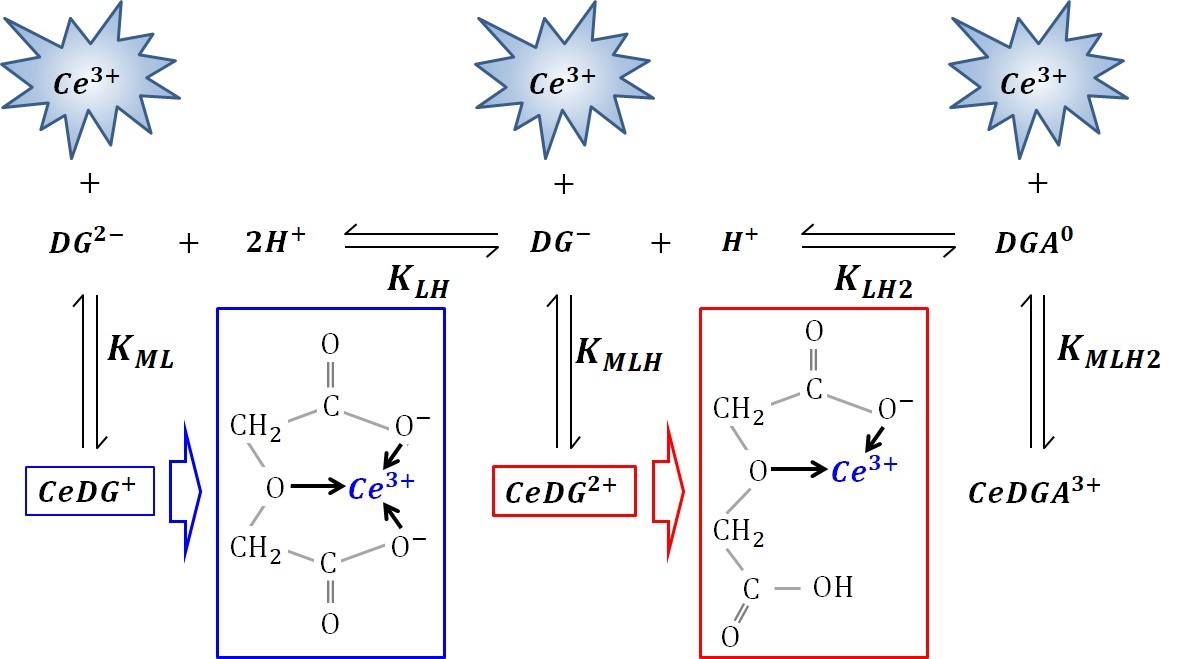
Interactions between hydrated Ce3+ and various carboxylates are of fundamental interest. Anomalously strong interactions with Ce3+ occur when adding diglycolic acid (DGA) into a Ce3+ aqueous solution, unlike various other carboxylic acids. The complex formation constants of Ce3+ with these acids are evaluated via absorption and emission spectra. Hydrated Ce3+ emits fluorescence with unity quantum yield; however, adding various carboxylates statically quenches the fluorescence when Ce3+‒carboxylate complexes form, because the fluorescence lifetime is constant irrespective of the carboxylate concentration. In the observed static quenching, the complex formation constants obtained from the absorption and emission spectra (Kabs and Kem) agree well. The binding Ce3+ by the conjugate Lewis bases, i.e., carboxylates is approximately inversely proportional to the pH. Adding DGA into the system also statically quenches the fluorescence, but far more efficiently, even in a much weaker solution. We rigorously deduce Kabs and Kem of Ce3+ with DGA without any approximation using comparable concentrations. Careful fittings provide equivalent Kem and Kabs values, and varying the pH and ionic strength confirms this equivalence is an inherent property of the Ce3+-DGA system. Lewis acid–base theory cannot explain DGA binds Ce3+ ~1000 times more strongly than the other carboxylates. This anomalously strong binding may be due to a chelate effect caused by the DGA’s central oxygen atom, which forms a five-membered ring with the conjugate Lewis bases of DGA; double chelate rings can also form, while bis-deprotonated DGA binds Ce3+, facilitated by the central oxygen. Therefore, DGA enables efficient quenching through the chelate effect when it binds Ce3+.